No to claims giving a “health” image to food products high in added sugars
Is it acceptable for food products to present a health image based on a few claims, while masking the reality of their very high sugar content (especially added sugars) by refusing to display the updated version of the Nutri-Score that allows consumers to know, for their health, that these products should not be consumed in large quantities or too frequently. Yet, this is exactly what is happening, with drinkable yogurts (Actimel, Activia, Danonino) and protein drinks (HiPro) manufactured by Danone.
Just looking at the packaging or the presentation websites of these products reveals what is highlighted and what is quite concealed:
- On one side, we can see and read: “Actimel: The small immunity gesture for good days. Actimel is a delicious shot rich in vitamin D and a source of vitamin B6 to take care of your immune defenses every morning. Billions of L.casei ferments…”; “Activia: contains Bifidus Acti Regularis, billions of probiotics that help you better digest lactose in case of difficulties, takes care of your digestive well-being, source of calcium that contributes to the normal functioning of digestive enzymes…”; “Danonino: Calcium + vitamin D: good fruits, milk, calcium, and vitamin D for normal bone growth in children, without artificial flavors, no colors, no preservatives”; “HiPro: 25g of protein, the ally of athletes”.
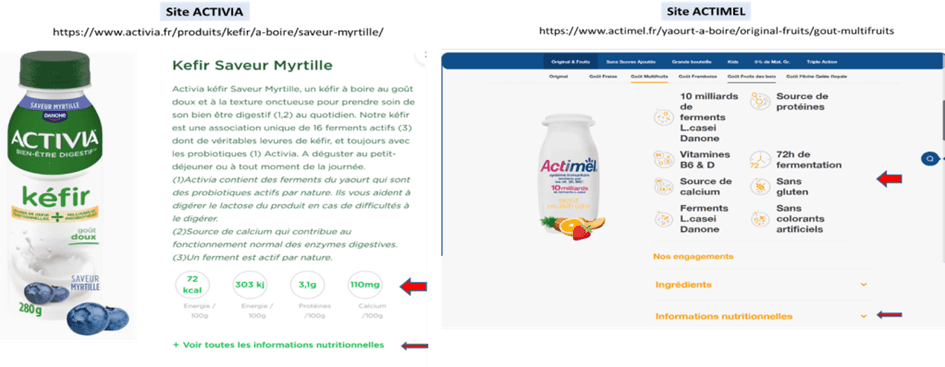
- On the other hand, however, the sugar content of these products is not visible (or very hard to see). Outside of the versions that specify “no added sugars,” which only contain the naturally occurring sugar in milk (lactose) at a rate of 4g/100 ml, they contain significant amounts of sugar, between 8 and 12 g/100 ml for the various Actimel available on the market. That is as much sugar as in a regular soda or flavored milk! The amounts of sugar are also 7 to 10 g/100 ml for drinkable Activia and 9.5 to 9.9 g/100 ml for drinkable Danonino. While this information is available on the packaging, it is in very small print, buried in the mandatory nutritional values table, placed nearly unreadably on the back of the packaging, while enticing promises are written in large letters on the front of the packaging and on the information or sales websites for these products.
The Actimel, Activia, and Danonino websites do not prominently display the sugar content of the different products in their general, easily accessible pages, while their protein and calcium contents, as well as their sources of calcium, vitamins B6 and D, and protein, are specifically highlighted in large print, along with the fact that they do not contain gluten or food dyes, and that they provide active ferments… You must click on “See all nutritional information” to find in the displayed nutritional table the carbohydrate content, including the total amount of sugars among many other data.
All of this raises questions about Danone’s willingness to provide real transparency regarding the nutritional composition of their drinkable yogurts, particularly concerning the unfavorable elements they contain, in order not to “tarnish” the image of these products in the eyes of health-conscious consumers. This seems to explain Danone’s refusal to display the new version of the Nutri-Score on these products, which would legitimately classify them in categories D or E, given their high sugar content (only the plain versions, with no added sugars, containing only the natural sugar from milk, remain classified as B, and those with low sugar content are classified as C).
The following figure shows what the Nutri-Score display would look like in its new version if Danone were willing to engage in transparency regarding the nutritional composition of its products (some examples):

This ambivalence (highlighting “health” arguments vs. masking information about sugar content) reaches a peak with the latest versions of Actimel, the “Actimel+ Triple Action,” launched by Danone in September 2024 (just few days after the announcement of their refusal to display the updated Nutri-Score developed by scientists). Here’s what can be read in large type on the packaging of these new products: “20 billion L.casei Danone ferments”; “this product helps you stay at the top of your form through 3 actions: supporting the immune system thanks to vitamin D; protecting cells against oxidative stress to provide antioxidant properties thanks to vitamin C; reducing fatigue through magnesium.”
In fact, these products prominently feature claims on their packaging that are not based on demonstrated health effects of the products themselves (which have never been properly tested or proven), but rather indirectly through potential effects of certain vitamins, minerals, and other constituents they have been fortified with. Moreover, the presentation of these elements is somewhat misleading, as the generic health claims on the packaging are not actually related to the famous Lactobacillus Casei ferments, which Actimel implies to be an original compound of interest, but rather to the fact that these products are enriched with vitamins B6 and D and magnesium (nutrients that can also be found in a balanced diet), which play roles in various physiological functions. While these general claims are indeed permitted in Europe for these vitamins and minerals, the demonstration of a beneficial health effect from the products to which they have been added has never been proven. Furthermore, the European Food Safety Authority (EFSA) has never recognized or authorized any health claims for Lactobacillus casei. The arguments supporting these effects have been deemed non-demonstrative (to the extent that Danone has not even sought to resubmit its case to the EFSA, raising questions about the confidence the company has in its own claims).
If the various health benefits claimed for these products are not scientifically recognized, the harmful effects of high sugar intake are well established. However, the different versions of these Actimel+ Triple Action (Peach/Passion and Strawberry/Pomegranate) contain, like several other Actimel already on the market, significant amounts of sugar (10.8 g of sugar/100 ml, with a substantial portion being added sugars), which is as much, if not more, sugar than in other sugary drinks: for example, classic Coca-Cola contains 10 g of sugar/100 ml, Capri-Sun contains 7 to 10 g of sugar/100 ml, and Oasis contains 7 to 9 g of sugar/100 ml, depending on the variety.

Claims regarding the micronutrients used in these products are authorized by European legislation dating back to 2006 (Regulation (EC) No 1924/2006 on nutrition and health claims). It is acknowledged that these nutrients play multiple physiological roles and that true deficiencies in these substances could have detrimental effects on immune system functioning and health more generally. However, in France, deficiencies in these nutrients are rare in the general population, although moderate deficiencies in vitamin D are observed in certain population groups. There is no evidence to suggest that additional intake of these nutrients among non-deficient individuals in the general population can provide real health benefits. The nutrients and other antioxidants touted on packaging are naturally present in fruits and vegetables (notably vitamin C) and in a multitude of minimally processed foods (whole grains, fish, legumes, nuts, etc.). Moreover, neither in France nor in Europe is there any official public health directive recommending the use of foods enriched with antioxidants. On the contrary, it is considered that this could mislead consumers, leading them to be falsely reassured, away from foods that are natural sources of these bioactive compounds within their original matrices, such as fruits and vegetables, the consumption of which is promoted by public health recommendations in France and internationally.
Many foods that promote health (fruits and vegetables, whole grain products, fish, etc.) without being enriched are important natural sources of these vitamins and minerals (and many others). These are the foods that are the focus of public health recommendations for the population, not sugary drinkable yogurts or protein drinks.
Regarding the ability to promote claims on products that do not have a favorable nutritional composition, it is interesting to note that Article 4 of Regulation (EC) No 1924/2006 on claims stipulated that the European Commission should define, before the end of 2009, nutritional profiles (including sugar, salt, fat, saturated fatty acids, and trans fatty acids content) to characterize products that have a favorable nutritional profile allowing for the presence of claims (for instance, those made by Actimel regarding immune defenses or fatigue). The aim was to prevent a claim from masking an unfavorable nutritional composition of a food, which could mislead consumers. Unfortunately, due to pressure from agribusiness lobbies, and despite this regulation being adopted more than eighteen years ago by the European Parliament, it remains unimplemented in the absence of a definition of what this nutritional profile would be at the European level! This explains why Danone can still capitalize on claims on the packaging of Actimel today, despite their very high sugar content. This situation is completely unacceptable in terms of public health.
The health promises of these products therefore appear primarily as pure marketing arguments, which can be seen as even more confusing for consumers as they do not come with valid information concerning the actual overall nutritional composition of these foods (unfavorable due to their high sugar content) that the Nutri-Score would provide in its updated and scientifically validated version. Confusing, even misleading, this strategy seems effective in business terms. The market for drinkable yogurts is rapidly expanding in France and worldwide. Activia, Actimel, and Danonino drinks are flagship products in Danone’s portfolio. Giving a “health” appeal to these products allows for attracting a wider audience and standing out from competitors. And this apparently works! Actimel is currently consumed by 4 million households in France (and 32 million worldwide) and generates revenue of over €101 million with more than 13% growth in a year. Sales of Actimel units are even up by 2%, a figure even more interesting given that overall food consumption is slowing. Moreover, these well-marketed products have particularly interesting profitability. The price per kilo of Actimel is at least twice that of traditional yogurt forms marketed by the brand.
Furthermore, it should be kept in mind that Actimel products are ultra-processed foods (classified as NOVA 4) containing many additives and other industrial ingredients (dextrose, modified starches, citric acid, flavors, thickeners, sweeteners, etc.).
Finally, unlike solid yogurts, which are generally consumed as part of a meal, drinkable yogurts are positioned in the snack category and are therefore potentially consumed outside of meals (they can be considered liquid snacks). They have been designed for convenience (no spoon needed), and their flavored (sweet) forms are particularly appealing, especially to children and adolescents. All these factors encourage their consumption outside of meals and throughout the day. The highlighting of claims suggests that they have nutritional and/or health benefits, whereas, from a health perspective, it is advisable to limit consumption of the sweeter varieties.
Overall, promoting arguments that aim to present a health image for products while concealing useful information about their high sugar content, which is detrimental to health, does not help consumers understand how to use these products appropriately. It is essential to evolve legislation to protect consumers from aggressive marketing employing this strategy. We cannot accept that marketing arguments alone take precedence over consumer health and public health.
This text is signed by :
Pr Serge Hercberg, Professeur des Universités – Praticien Hospitalier (PUPH), Professeur émérite de Nutrition, Université Sorbonne Paris Nord
Dr Mathilde Touvier, Directrice de Recherche INSERM, Directrice de l’Equipe de Recherche en Epidémiologie Nutritionnelle/CRESS
Pr Chantal Julia, Professeur des Universités – Praticien Hospitalier, Professeur de Nutrition (PUPH), Département de Santé Publique Group Hospitalier de Paris Nord
Dr Mélanie Deschasaux-Tanguy, Chargée de Recherche INSERM Nutrition, EREN/CRESS
Pr Boris Hansel, Professeur des Universités – Praticien Hospitalier (PUPH), Endocrinologie-diabétologie et nutrition, Université Paris Cité, Hôpital Bichat Claude-Bernard, Paris
Pr Claire Carette, Professeur des Universités – Praticien Hospitalier (PUPH), Médecin spécialiste du Diabète et de l’Obésité, Professeure de Nutrition, APHP, Université Paris Cité
Pr Pierre Dechelotte, Professeur des Universités – Praticien Hospitalier, PUPH en Nutrition, CHU et Université de Rouen, UMR INSERM1073
Pr Pierre Henri Ducluzeau, Professeur des Universités – Praticien Hospitalier, PUPH, Diabétologue-Nutritionniste, CHU de Tours
Pr Roberto Mallone, Professeur des Universités – Praticien Hospitalier, PUPH Université Paris Cité et Diabétologie Hôpital Cochin, Paris
Pr Nathalie Le Moullec, Professeur des Universités – Praticien Hospitalier, Nutrition, CHU La Réunion
Pr Jean Claude Desport, Professeur Emérite de nutrition, Centre de Ressources en Nutrition (CERENUT), Nouvelle Aquitaine, Isle, Inserm U1094/IRD U170, Faculté de Médecine, Limoges
Dr Pilar Galan, Epidémiologiste de la nutrition ; Directrice de Recherche honoraire INRAE, EREN/CRESS
Dr Muriel Coupaye, Endocrinologue, diabétologue et nutritionniste, Hôpital Louis- Mourier, Colombes
Dr Vanessa Cottet, Maitre de Conférence des Universités-Praticien Hospitalier en Nutrition, UFR des Sciences de Santé Dijon, CHU Dijon-Bourgogne, INSERM U1231/CIC1432
Dr Valentina Andreeva, enseignant-chercheur en nutrition, Université Sorbonne Paris Nord, EREN/CRESS
Ghislain Grodard-Humbert, Président de l’Association Française des Diététiciens Nutritionnistes (AFDN)
Pr Jean-Claude Carel, , Professeur des Universités – Praticien Hospitalier Professeur de Pédiatrie, Université Paris Cité, Service d’Endocrinologie Diabétologie Pédiatrique & INSERM NeuroDiderot, Hôpital Universitaire Robert-Debré, Paris
Pr Noël Peretti, Professeur des Universités – Praticien Hospitalier, Chef de service Nutrition Pédiatrique, Hospices Civils de Lyon
Dr Arnaud De Luca, Pédiatre nutritionniste, CHU Tours, Responsable du Centre Spécialisé de l’Obésité (CSO) de Tours, Secrétaire Général de la Société Francophone, Nutrition Clinique et Métabolisme (SFNCM)
Pr Daniel Floret, Professeur des Universités – Praticien Hospitalier, Professeur Emérite de Pédiatrie, Université Claude Bernard Lyon1
Pr Rachel Reynaud, Professeur des Universités – Praticien Hospitalier, Service de Pédiatrie Multidisciplinaire, CHU Timone Enfants Marseille, Centre Spécialisé Obésité Sévère et Compliquée PACA Ouest
Dr Véronique Nègre, Médecin des hôpitaux – Pédiatre, Coordination médicale des Centres Spécialisés Obésité PACA, Présidente Association pour la Prévention et la prise en charge de l’Obésité en Pédiatrie
Pr Brigitte Delemer, Professeur des Universités – Praticien Hospitalier, PUPH Endocrinologie, Diabète, Maladies Métaboliques au CHU de Reims
Pr Emmanuel Cosson, Professeur des Universités – Praticien Hospitalier, PUPH en Endocrinologie et Métabolisme, APHP-HUPSSD
Pr Philippe Chanson, Professeur Émérite Université Paris-Saclay, Service d’Endocrinologie, Hôpital Bicêtre, Assistance Publique Hôpitaux de Paris
Dr Annick Fontbonne, médecin épidémiologiste (diabète/obésité), chercheuse INSERM retraitée
Dr Yves Martin-Prevel, Directeur de Recherche à l’IRD, Epidémiologiste en nutrition de santé publique, Montpellier
Pr Didier Courbet, Professeur de sciences de l’information et de la communication, Université Aix-Marseille
Pr Rebecca Shankland, Professeur de psychologie, Université Lyon 2, Membre de l’Institut Universitaire de France
Dr Stéphane Besançon, Directeur ONG Santé Diabète et Professeur associé en global health au Conservatoire National des Arts et Métiers
Dr Pierre Senesse, Responsable de l’Unité Transversale de Nutrition Clinique, Institut du Cancer de Montpellier
Pr Michel Narce, Professeur Émérite, Physiologie de la Nutrition; Inserm/Université Bourgogne Europe
Dr Alice Grenier, Médecin coordonnatrice Centre Spécialisé Obésité (CSO), CHU de Bordeaux et Réseau de Prévention et de Prise en charge de l’Obésité Pédiatrique (RéPPOP) Aquitaine
Dr Amandine Diserbo, Médecin coordonnateur Prévention et Prise en charge de l’Obésité Pédiatrique, RéPPOP 38
Pr Franck Carbonnel, Professeur des Universités – Praticien Hospitalier, Chef du Service de Gastroentérologie, Hôpital de Bicêtre, Assistance Publique- Hôpitaux de Paris, Université Paris Saclay, INSERM U1018
Pr David Laharie, Professeur des Universités – Praticien Hospitalier, Professeur de Gastroentérologie CHU de Bordeaux
